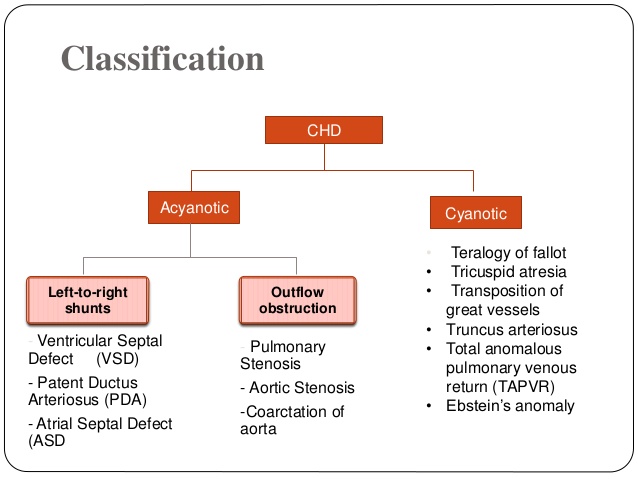
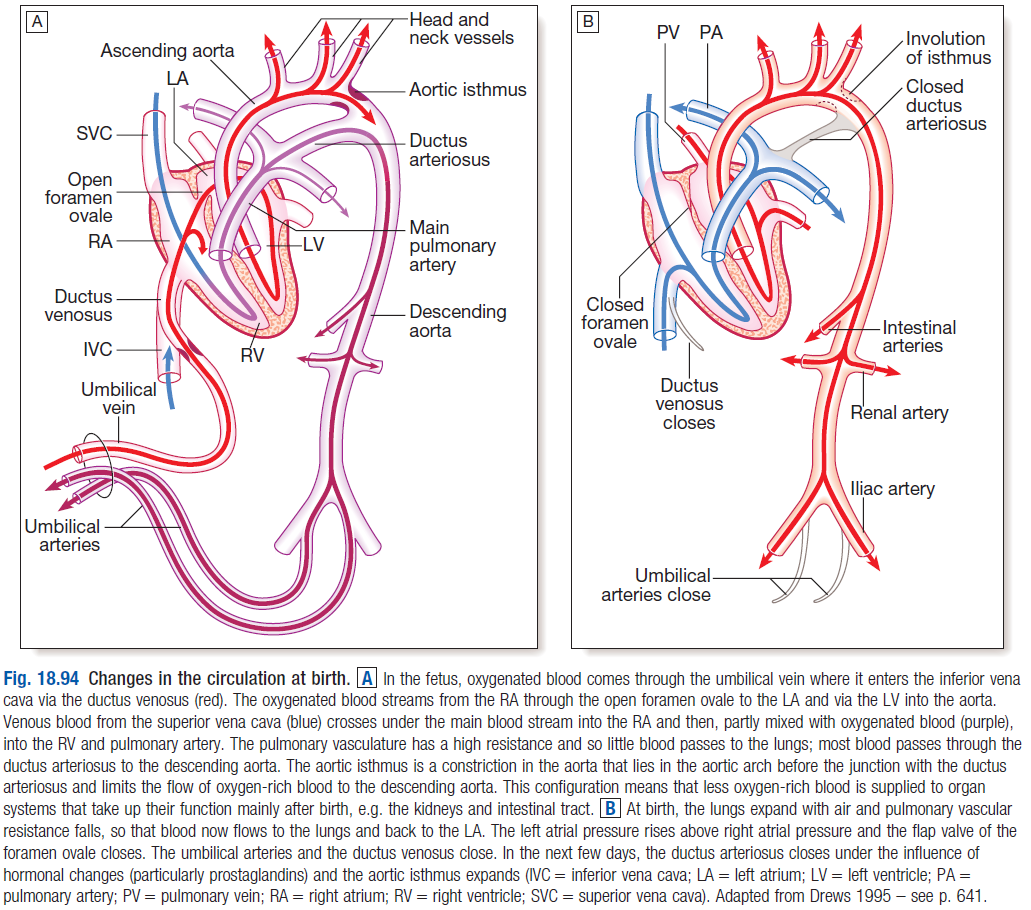
1. FETAL CIRCULATION
2. VENTRICULAR SEPTAL DEFECT
Etiology
- Due to incomplete separation of ventricles
- Acquired – acute MI, trauma
Pathophysiology
- Left to right shunt causes more blood to enter from LV to RV during systole
- From RV it enters the lungs and then back to the LV (via pulm veins and LA)
- Leads to volume overload of the LV – leads to high output HF
- ↑vol in the RV outflow tract can cause pulmonary congestion
Clinical features
- Pansystolic murmur – due to flow from LV to RV
- Small defect = loud murmur
- Large defect = soft murmur
- Pale, underweight, irritable child
- Failure to gain weight
Diagnosis
- Echo – gold standard
Management
- Small VSDs don’t require tx
- Drugs – loop diuretics, digoxin, ACEi
- Surgery – for persisting failure
- Percutaneous or open heart surgery
- Eisenmenger syndrome is avoided by monitoring for signs of PHTN
3. PERSISTENT DUCTUS ARTERIOSUS (PDA)
Etiology
- Risk factors – maternal rubella, premature birth, Downs syndrome
Pathophysiology
- During fetal life, before lungs begin to function, most blood from the pulmonary artery (PA) passes through the DA into the aorta
- Normally, DA closes soon after birth – due to ↑PaO2 and ↓prostaglandins
- PDA leads to a continuous arterio-venous shunt (left to right shunt) – since Paorta > Ppulmonary artery
- Therefore pulmonary blood flow is excessive
- 50% of the LV output is recirculated through the lungs, which leads to an increase in the work of the heart
Clinical features
- No symptoms if the shunt is small
- Growth and development stunting if shunt is large
- Poor feeding, SOB on feeding
- No disability in infancy, but later in life presents as dyspnoea and HF
- Continuous murmur
Persistent ductus with reversed shunting
- If pulmonary vascular resistance increases, PA pressure can rise until it exceeds aortic pressure
- The shunt can be reversed – causing Eisenmenger’s syndrome
Diagnosis – echo is diagnostic
Management
- Cardiac catheterisation – coil inserted to close the PDA
- In premature infants – indomethacin (PG inhibitor) can stimulate closure
4. ATRIAL SEPTAL DEFECT
Etiology
- More common in pts with – Downs syndrome, Ebstein anaomaly , fetal alcohol syndrome
- MC in females
- 3 main types of ASD
- Ostium secundum (75%) – located in fossa ovalis in the mid septum
- Ostium primum – located in the lower part of the atrial septum
- Sinus venosus defect – located near SVC
Pathophysiology
- Left to right shunt causes blood to flow from the LA to the RA
- This extra blood from the LA causes a vol overload of the RA + RV
- Can result in right heart overload and dilation
- Increased pulmonary flow causes PHTN
Clinical features
- Asymptomatic if ASD is small
- Recurrent chest infections, wheezing
- Heart failure
- Split S2
- Murmur
Diagnosis
- CXR – prominent pulmonary artery
- Echo – shows jet of blood from LA to RA; RVH; PA dilation
- ECG – secundum (RAD, RBBB), primum (LAD)
Management
- Surgical closure – implantable closure devices during cardiac catheterization
5. COARCTATION OF AORTA
Etiology
- Associated with Turner’s syndrome; bicuspid aortic valve
- Most commonly affects thoracic aorta
Pathophysiology
- Narrowing of the aorta in the region where ductus arteriosus inserts into the aorta – i.e. the isthmus
- Causes severe obstruction of blood flow in the descending thoracic aorta
- Encourages formation of collateral channels from the periscapular and intercostals arteries
- Decreased renal perfusion can lead to systemic HTN
Clinical features
- Headaches, chest pain, leg claudication
- Heart failure in infancy (not common in later age)
- HTN in upper limbs, hypotension in lower limbs
- Delayed pulse in legs – radiofemoral delay
- Bruits – from collateral circulation
- Renal failure
Diagnosis
- CXR – 3 sign
- Doppler echo – shows coarctation
- ECG
Management
- Neonates – surgical repair
- Older children/adults – balloon dilation, stenting
6. TETRALOGY OF FALLOT
Etiology
- Associated with maternal rubella, maternal alcohol, diabetes
- Downs syndrome
- MCC of cyanotic heart disease
Pathophysiology
- 4 features
-
- Large VSD
- Pulmonary valve stenosis – RV outflow obstruction
- Overriding aorta – aorta is positioned over the VSD instead of the LV. Receives blood from both LV+RV
- RV hypertrophy
- Symptoms depend on the degree of pulmonary stenosis – cyanosis develops due ↑right sided pressures
- Leads to right to left shunt
Clinical features
- Cyanosis – when RVP is equal to or exceeds LVP, leading to formation of right>left shunt
- Dyspnoea on feeding/crying
- Failure to thrive
- Fallot spells – episodes of severe cyanosis triggered by crying/feeding/distress
- Children squat instinctively during spell – increases SVR and allows temporary reversal of shunt
Diagnosis
- ECG – RVH
- CXR – small PA, boot shaped heart
- Echo – diagnostic
Management
- Surgical repair of pulmonary stenosis and closure of VSD – before 5 years of age
- Blalock-Taussig shunt – anastomosis created between PA and subclavian artery
- Improves blood flow through pulmonary circulation
7. TRANSPOSITION OF THE GREAT ARTERIES (TGA)
- RV is connected to the aorta and LV is connected to the PA
- Incompatible with life as blood circulates in 2 parallel circuits
- Deoxygenated blood from the systemic veins passes into the right heart and then back into the systemic circulation through the aorta
- Oxygenated blood from the pulmonary veins passes through the left heart and back into the lungs through PA
- Babies are born cyanosed
- A coexisting ASD, VSD or PDA can delay diagnosis – as the shunt allows mixing of blood
- Atrial septostomy performed as temporary solution until definitive repair
- Rashkind balloon inserted to dilate the foramen ovale
- Atrial switch – definitive repair
- Performed in first 2 weeks of life
- Aorta is reconnected to the LV and PA connected to the RV and coronary arteries are re-implanted