- MDS – decreased number of morphologically abnormal cells that often don’t function properly
- Clonal proliferations of hematopoietic stem cells
- Many cells die in the BM – ineffective hematopoiesis
- Therefore MDS are characterised by cytopenias, abnormal morphology, impaired function of cells
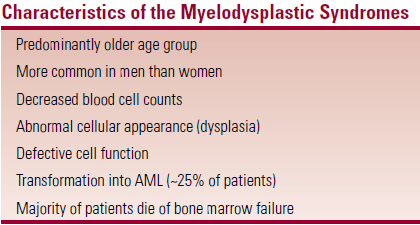 Majority of pts die of BM failure
Majority of pts die of BM failure- Aggressive forms of MDS resemble AML (distinction can be difficult)
Classification
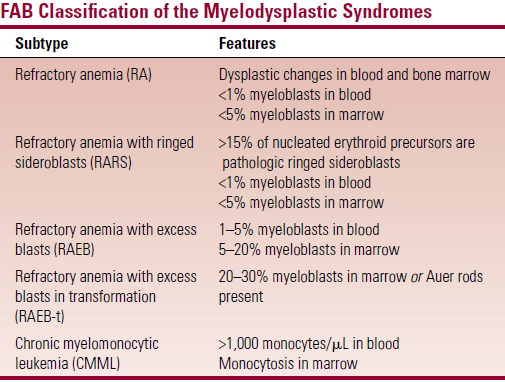
- FAB – based on number of myeloblasts in blood and BM
- But has limited prognostic ability
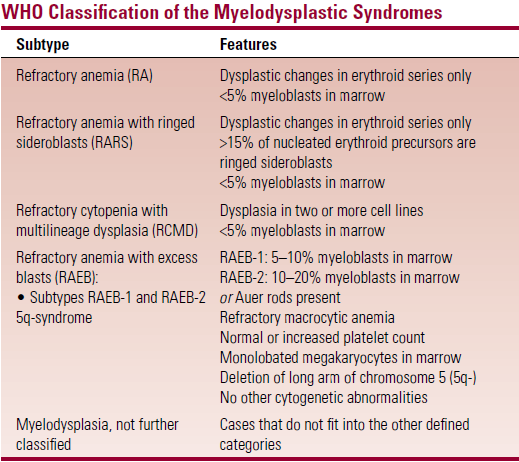
- Modifications in the WHO classification
- RA and RARS are limited to cases with dysplastic changes restricted to erythroid series only (patients have a longer survival)
- RCMD – <5% myeloblasts in BM but with dysplastic changes in two or more cell lines (pts have shorter survival)
- RAEB – is divided into RAEB-1 and RAEB-2 depending on myeloblast count and presence/absence of Auer rods
- RAEB-t – is eliminated in the WHO system
- Cases that fit in this category in FAB are now classified as AML
- Myelodysplasia arising in patients who previously received chemo for a malignant disease is now classified as AML
- Patients with characteristic AML translocations [t(8;21), t(15;17) or inversion (16)] are classified as AML even if blast count is low
- CMML is moved to a new disease category – the ‘myelodysplastic/myeloproliferative syndromes’
Epidemiology
- MC in older men
- Etiology mostly unknown
- ↑risk with exposure to ionizing radiation, chemotherapy, benzene
Clinical features
- 50% detected as incidental findings
- Clinical variants
- Symptoms are related to cytopenias – fatigue due to anaemia is MC
- History of multiple infections
- Patients may have petechiae, purpura, mild splenomegaly
- Marked splenomegaly is more indicative of a myeloproliferative disorder than MDS
Diagnosis
- Most patients are anemic
 Normal/high MCV
Normal/high MCV- Leukopenia, thrombocytopenia, pancytopenia
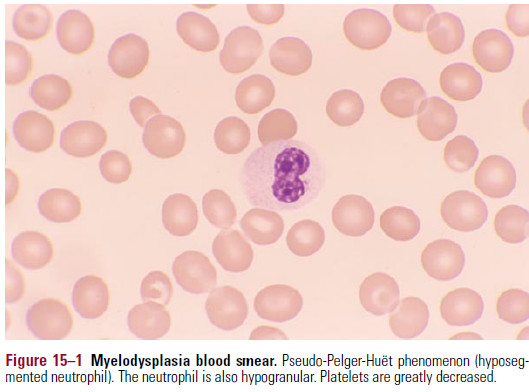
- Blood smear
- Anisocytosis of er
- Neutrophils have abnormal segmentation and are hypogranular
- Pseudo Pelger-Huet anomaly
- Decreased MPO activity
- Bone marrow
- Hypercellular
- Er precursors are enlarged
- Er nuclei appear karyorrhexic – nuclear fragmentation
- Granulocytes have nuclear-cytoplasmic asynchrony
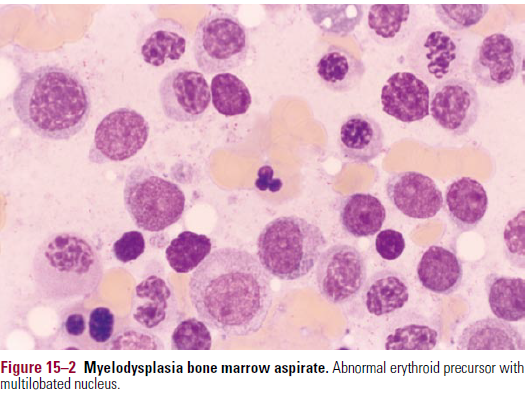 Increased myeloblasts
Increased myeloblasts- May be presence of ringed sideroblasts
- Nucleated erythroid precursors with granules adjacent to the nucleus (on Prussian blue stain)
- Cells in abnormal locations
- E.g. er precursors located next to bone trabecula (they are normally located away from bone)
Cytogenetics
- Crucial in prognosis
- Partial/complete chromosome deletions
- MC in chromosomes 5,7,8,20
Disease course
- Highly variable
- RA + RARS – low grade, prolonged survival, low rate of transformation to AML
- RAEB and RAEB-t – high grade
- RCMD – intermediate
- MCC of death – infection, hemorrhage, leukemic transformation
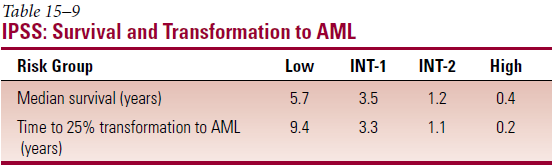
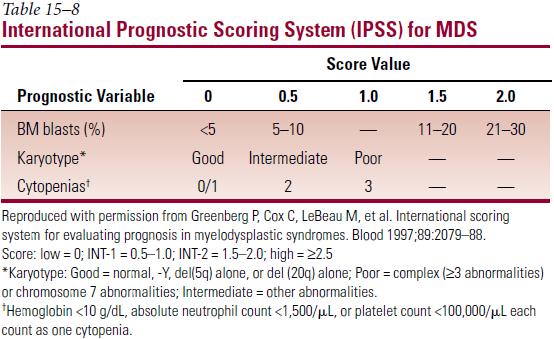
- Most important prognostic factors – age; % myeloblasts in BM; no. of cell lines with cytopenia; cytogenetics
- International Prognostic Scoring System (IPSS)
Treatment
- Older patients with comorbidities unable to tolerate aggressive therapies
- Control of symptoms is primary goal
- Supportive – transfusions, antibiotics for infection
- Pyridoxine – for pts with RARS only
- Hematopoietic growth factors (erythropoietin, G-CSF, GM-CSF (pegfilgastrim)
- Chemotherapy – low response rates compared to pts with de novo AML
- Cyclosporin, Azacitabine, Lenolidamide (5q syndrome)
- BMT – only curative. Pts with RA + RARS have best response
